Why Does Distillation Produce Pure Water and Filtration Does Not
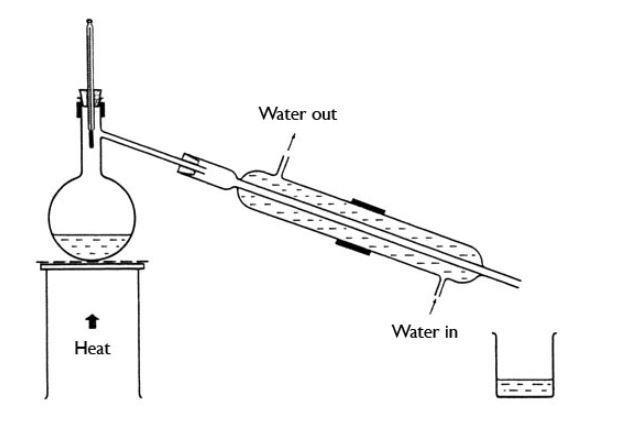
The water which is going to evaporate from the distilling flask is cooled by using a condenser and then collected in a receiving flask. - The below picture depicts the process of simple distillation clearly.

U5thc Describe 1 Flashcards Quizlet
The equipment would also take a large amount of space for a household.
. Filtration simply involves passing the mixture through a filter which separates out. Water from rivers lakes and the sea has to be treated to make it safe to use and drink. This results in the contaminants.
Produces consistently high quality water. Unlike some organic contaminants which usually transfer into condensed water inorganic minerals such as sodium and chloride will not. The steam rises from the water leaving virtually all other contaminants behind then we cool the steam down into pure water.
Why does distilled water do not conduct electricity. Some of these organic compounds might actually become more concentrated due to distillation. This is why distillation is usually the go-to method of filtration for most people.
Another disadvantage of distilled water is that it can be very acidic low pH thus it should be contained in glass. Distillation does not always produce pure water. Why does distillation produce pure water but filtration doesnt.
- The water which is collected in a receiving flask is pure water. Why does distillation produce pure water but filtration does not. Depending on the contamination filtration may work better.
See full answer below. When the water boils it evaporates and this evaporated water is then captured and passed through a system of tubes into a separate container and removed from the heat source. - This is how we can separate pure water from seawater.
Distilled water is a pure form of water which does not contain any solute in it therefore it cannot conduct electricity because it does not contain ions while rain water contains dissolved salts and acids which dissociates in ions and conducts electricity. During distillation a heat source is employed to vaporise the water separating the pure water molecules and the contaminants with a higher boiling point. How does distillation seaparte a mixture of different liquids.
There is no drop in quality over time. This is called a distillation water treatment system. It is becaise filtration only seperates the large particles particles larger then the filter paper.
Why does distillation produce pure water but filtration doesnt. Distillation is great at removing metals with a low vapor pressure and ionic solids. Likewise distillation cannot remove volatile organic compounds from your water.
Distillation produces extremely consistent results. Distillation is also costly as it requires large amounts of energy and water and is very slow to produce clean water. Sometimes the distilling process actually adds contaminants that werent originally present from the glassware or metal components.
Share this link with a friend. Distillation produces a much more pure substance than filtration. Different methods are used to do this such as sedimentation filtration and chlorination.
You can also decide to heat the salty water to boiling point using temperature change then condensing and collecting the vapor to produce pure water. However no method can provide you with 100 purified water. If there are other components that also distill they will pass over with the water.
Filtration only seperates the solids from the liquids but distillation seperates all liquids solids or other liquids Explain why the water in is near the exit of the condenser.
Why Does Simple Distillation Not Give Pure Compounds Quora
Recovering Water From A Solution Using A Condenser Experiment Rsc Education

No comments for "Why Does Distillation Produce Pure Water and Filtration Does Not"
Post a Comment